Please click the button below to go to our email login page
|
Thoughts on Wnt/β-catenin Reaches New Heights! Xiaohuan Guo’s team from Tsinghua University Unveils A Novel Mechanism Driving Intestinal InflammationWnt is originally derived from integrase-1 in mouse breast cancer and wingless in drosophila. Since these two genes and functional proteins share high similarity, researchers have fused the terms into “Wnt”. The Wnt signaling pathway includes both non-classical and classical pathways. The classic Wnt pathway, also known as the Wnt/β-catenin pathway, involves nuclear translocation of β-catenin and activates target genes through TCF/LEF transcription factors. So what are the design ideas for the Wnt/β-catenin signaling pathway?
Next, we will share a literature with an impact factor of 14.7, published in the journal Nature Communications in the 1st district of the Chinese Academy of Sciences. We hope it can bring different inspiration to everyone.
Research background
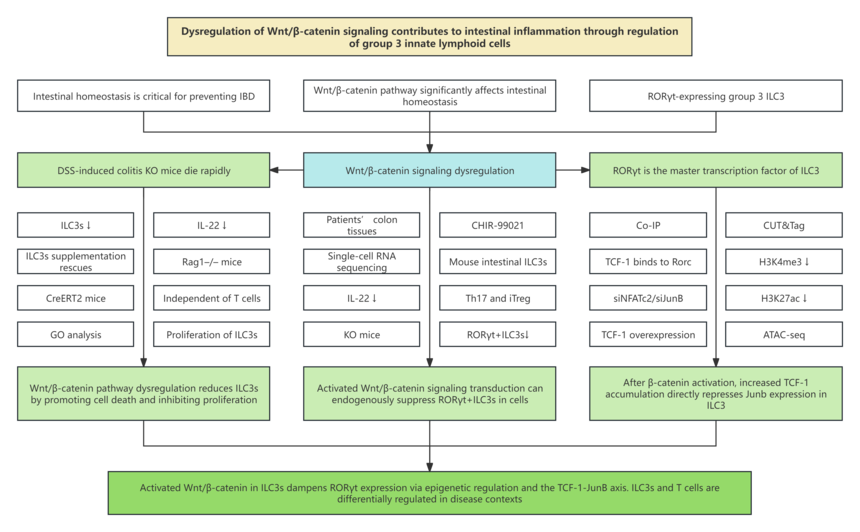
1. Intestinal homeostasis is indispensable for maintaining host health and preventing IBD (IBD) and colorectal cancer (CRC). 2. Different signaling pathways including the Wnt/β-catenin pathway greatly impact intestinal homeostasis. 3. Innate lymphoid cells (ILCs), incorporating ILC1, ILC2 and ILC3, have similar characteristics to their CD4+T helper cell counterparts and play important roles in various tissues. 4. RORγt-expressing group 3 ILCs (ILC3s) particularly exist in the intestinal mucosa and gut-associated lymphoid tissues. 5. Yet how inflammation or tumorigenic intestinal microenvironment affects ILC3s remains largely undefined.
Technical route
Research results 1. Wnt/β-catenin signaling transduction is dysregulated in ILC3 from IBD and CRC patients 2. Activation of Wnt/β-catenin signaling transduction differentially affects ILC3s and T cells in vitro 3. Genetic activation of β-catenin causes ILC3 deficiency in vivo 4. Activated Wnt/β-catenin signaling transduction in ILC3s increases susceptibility to colitis 5. Activation of Wnt/β-catenin signaling transduction affects cell survival and proliferation in ILC3s 6. TCF-1 cannot directly suppress RORγt expression in β-catenin-activated ILC3s 7. Activated β-catenin signaling transduction influences ILC3s through epigenetic regulatory mechanisms 8. NFATc2 and JunB mediate RORγt expression in ILC3s 9. TCF-1 binds to the Junb promoter and represses JunB expression in ILC3s
Conclusion This study observed an increase in β-catenin in intestinal ILC3 in patients with IBD and colon cancer compared to healthy donors. In contrast to promoting RORγt expression in T cells, the activated Wnt/β-catenin signaling transduction in ILC3 inhibits RORγt expression, proliferation, and function through epigenetic reprogramming and regulation of the TCF-1-JunB axis, leading to ILC3 deficiency and subsequent intestinal inflammation in mice. Activated β-catenin and its interacting transcription factor TCF-1 cannot directly inhibit RORγt expression, but instead alter global chromatin accessibility and inhibit JunB expression, while JunB expression is crucial for RORγt expression in ILC3. In summary, the research findings indicate that dysregulated Wnt/β-catenin signaling transductionimpairs intestinal ILC3 through regulating TCF-1/JunB/RORγt, further disrupts intestinal homeostasis, and promotes inflammation and cancer. |