Please click the button below to go to our email login page
|
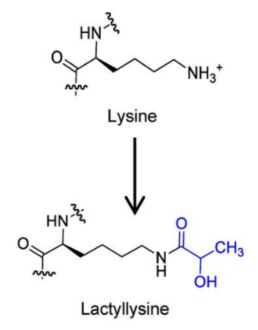
Protein post-translational modification---LactylationWhat is lactylation? Lactation refers to the linking of a lactate group to a lysine residue in the peptide chain of proteins, which can change the function and stability of proteins. Lactation has been found as a new histone modification in 2019, and later become a hot spot in research about protein post-translational modification, which has a broad research prospect in tumor and immune fields.
Lactation can modulate epigenetic phenomena and thus affect the
transcriptional control of genes. For example, lactate, accumulated during cell
metabolism, can serve as a precusor substance to cause lactation of histone
lysine and to participate in homeostasis regulation of bacteria-infected M1
macrophages. Meanwhile, lactation can repair DNA damage. For instance, lactation
of pyruvate kinase M2 (PKM2) protein promotes repair of DNA from damage. In
addition, lactation can also affect the mechanical behavior of histone
interaction with DNA.
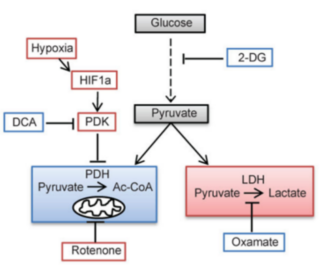
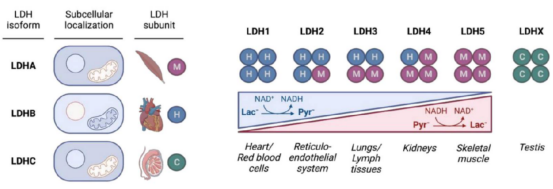
In the process of lactation, lactatedehydrogenase (LDH) is a key enzyme, which is a tetramer molecule with three isomers, namely muscle subunit (M), heart subunit (H), and testicle-specific subunit (C). M and C exist mainly in cytoplasm and partly in cell nucleus, and H is primarily present in mitochondria and cytoplasm. Different LDH isoenzymes have various affinity, expression and tissue specificity to the substrate. LDH1 is a homologous tetramer composed of four H subunits, and is preferentially localized in the heart and red blood cells. LDH2 is composed of three H subunits and one M subunit, mainly present in the reticuloendothelial system. The following isoenzymes are located in the lungs, lymphatic tissues, kidneys, skeletal muscles, and testes. H and M subunits are distributed in organisms based on specific metabolic needs of the tissues. H subunit depends on aerobic metabolic pathways and mainly exists in the heart. The myocardium requires sustained supply of energy which is catalyzed by LDH and maintains through conversion of lactate into pyruvate, together with reduction of oxidized coenzyme I (NAD+) to reduced coenzyme I (NADH). Besides, M subunit is abundant in skeletal muscle. During physical activity, LDH converts pyruvate into lactate and oxidizes NADH to NAD+.
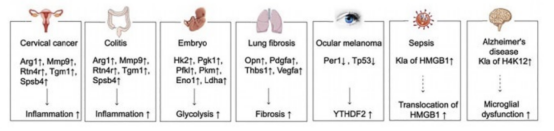
Lactation mainly occurs in specific residues of histones H3 and H4, with important sites including K9, K14, K18, and K23 of H3, as well as K5, K8, K12, and K16 of H4. Lactation is associated with multiple physiological and pathological processes, consisting of cell differentiation, mitochondrial membrane structure and function stability, etc., and meanwhile participates in the progression of diseases such as pulmonary fibrosis, tumors, cardiovascular diseases, and neurological disorders. The above figure unveils the interplay of lactation and inflammation-related genes in some diseases. In human cervical cancer and colitis, lactation leads to increases of expressions of inflammation-related genes, including dehydrogenase 1 (ARG1), matrix metalloproteinase 9 (MMP9), and reticulon 4 receptor (RTN4R). In embryos, it can upregulate the expression levels of glycolysis-related genes such as hexokinase-2 (HK2), phosphoglycerate kinase-1 (PGK1), and phosphofructose kinase-2 (PFK1). Also, upregulation of inflammation-related genes is detected in pulmonary fibrosis and melanoma of the eye.
|