Please click the button below to go to our email login page
|
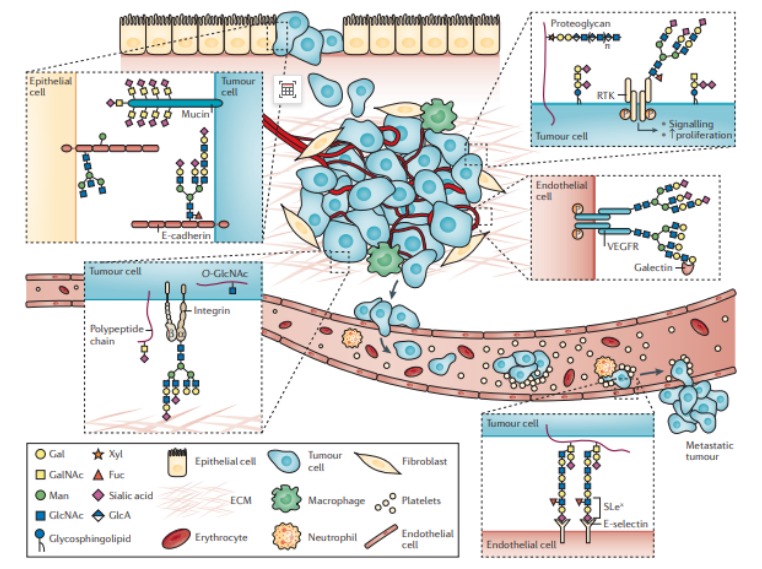
Let's learn deeply about the research design of protein glycosylation from one literatureProtein glycosylation is a process where carbohydrate chain molecules react with protein amino acid side chain active group to generate glycosidic bond under catalysis of glycosyltransferase, so as to link carbohydrate chain to protein. Protein glycosylation is one of the protein post-translational modifications with relatively high abundance and complex structure. Protein glycosylation plays a vital role in intracellular/intercellular signal transduction and immune regulation. Compared to normal cells, glycosylation of tumor cells may be accompanied by large-scale and multi-level changes, especially in the structure of carbohydrate chain which can result in incomplete carbohydrate chain synthesis or abnormally increased synthesis, and such aberrant glycosylation may incur the development of cancer.
Generally, the research about protein glycosylation mainly includes four steps: 1) Determining whether target proteins exist glycosylation 2) Identifying glycosylation sites 3) Dissecting the effect of glycosylation on target proteins 4) Exploring the biological function of target protein glycosylation
The following is a literature helping us to learn more about the process of protein glycosylation.
This literature was published in Nature Communications in 2021, with an IF of 16.6.
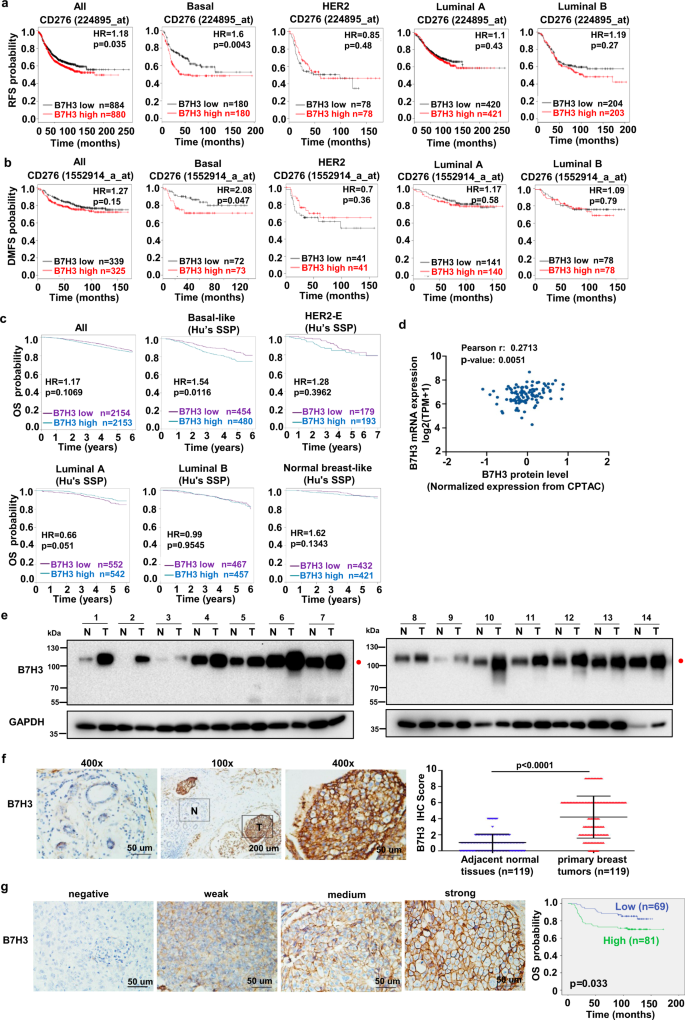
B7H3 is a highly glycosylated protein and an attractive target for cancer immunotherapy. Hence, researchers focused on the clinical significance of B7H3 up-regulation in triple negative breast cancer (TNBC), and confirmed glycosylated B7H3 up-regulation is related to TNBC progression and is a biomarker for poor prognosis.
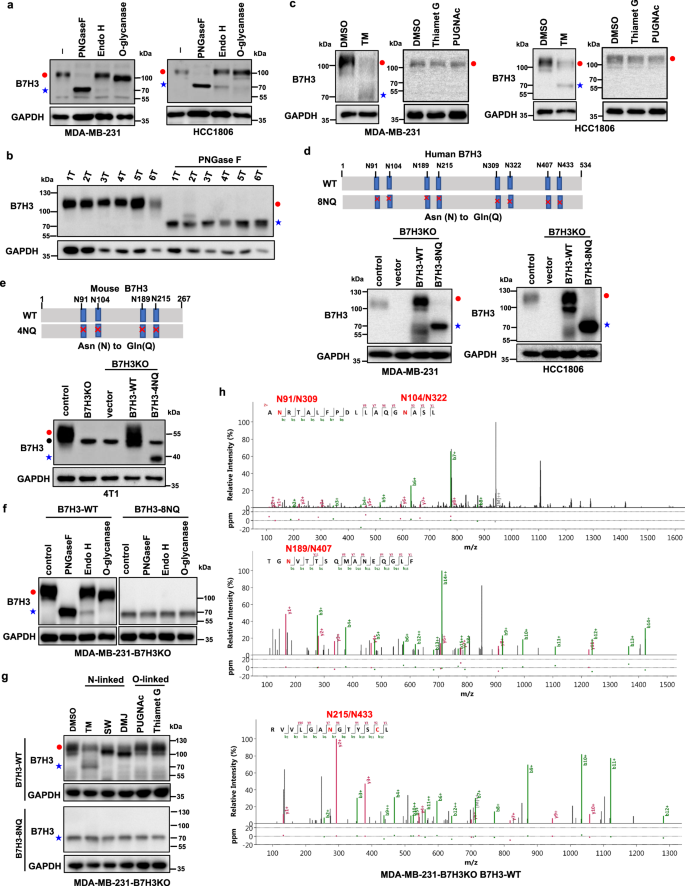
Then, through treatment of TNBC cell lysates with glycosidase and glycosylation inhibitors, researchers identified eight N-glycosylation sites (Asn positions 91, 104, 189, 215, 309, 322, 407, and 433) of B7H3 in TNBC cells.
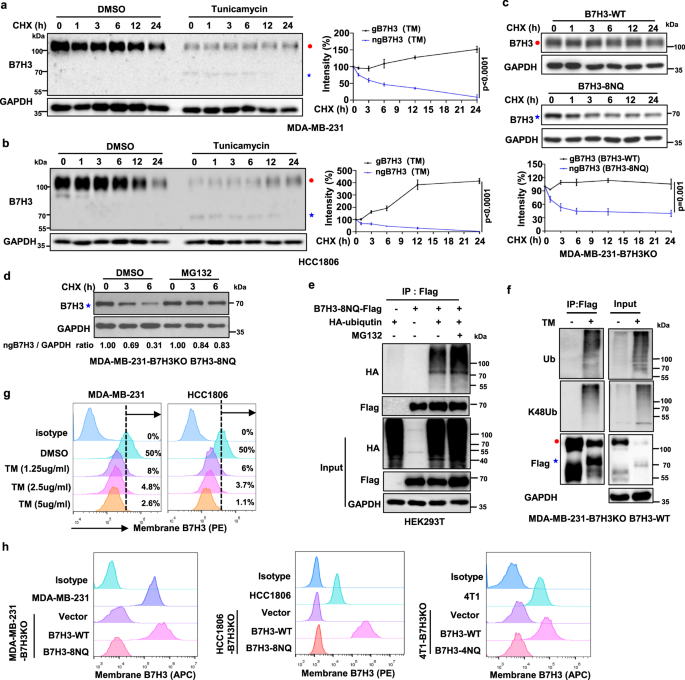
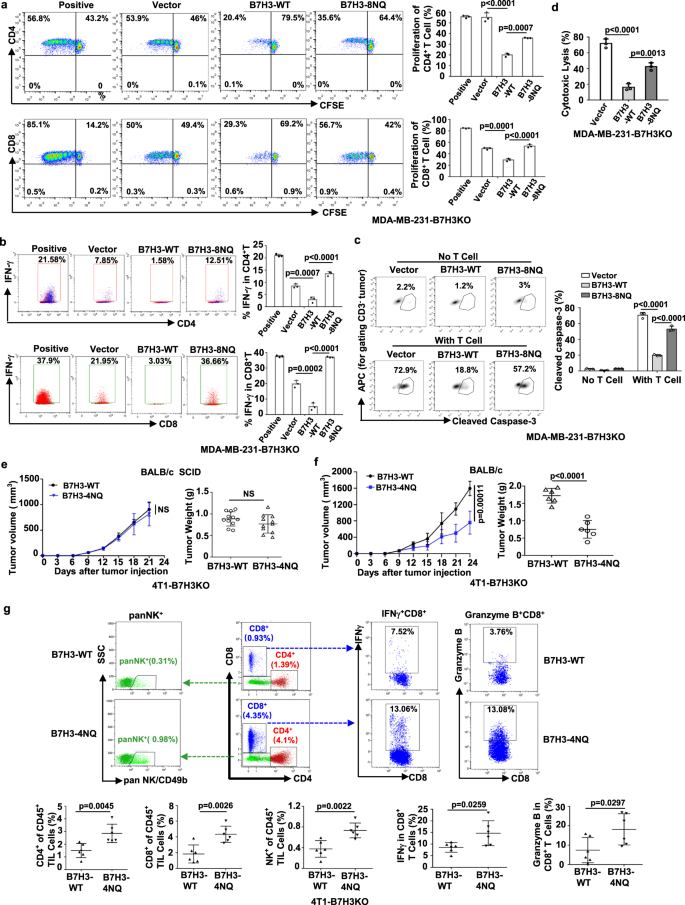
The subsequent exploration about the impact of N-glycosylation of B7H3 upon TNBC cell immune response suggested that N-glycosylation of B7H3 has an association with B7H3 protein stability and its stable expression on the cell surface and can suppress TNBC cell immune response.
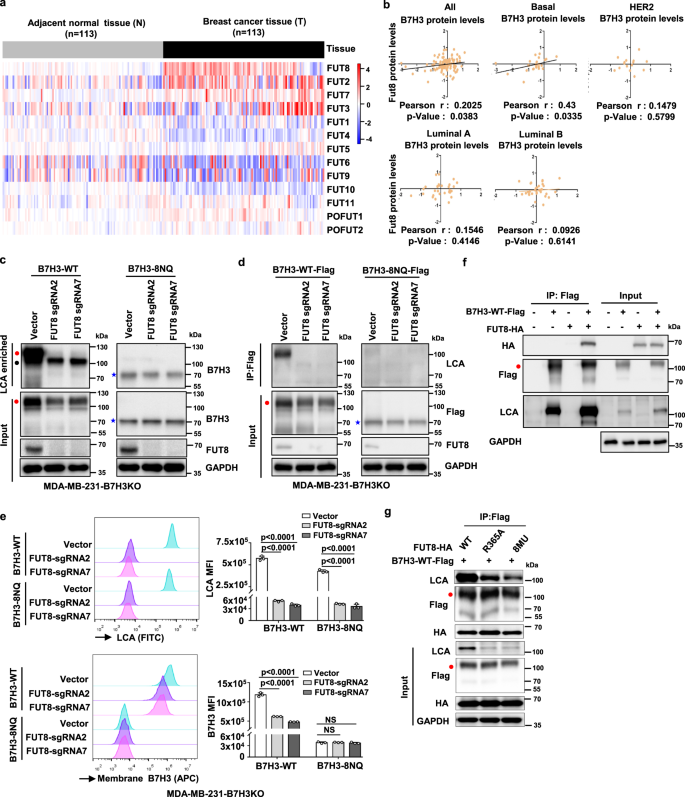
Due to the key role of B7H3 glycosylation in immunosuppressive activity, researchers sought to identify the mechanisms underlying aberrant B7H3 N-glycosylation regulation in TNBC cells and conducted relevant assays.The analysis results of TCGA revealed that FUT8 is one of the most significantly up-regulated fucosyltransferase genes in breast cancer tissues. To verify whether FUT8 can affect N-glycosylation of B7H3, a series of experiments were designed. The results indicated that FUT8 can maintain core fucosylation of B7H3 at N-glycans and its stability and augmentative effect of cell-surface expression.
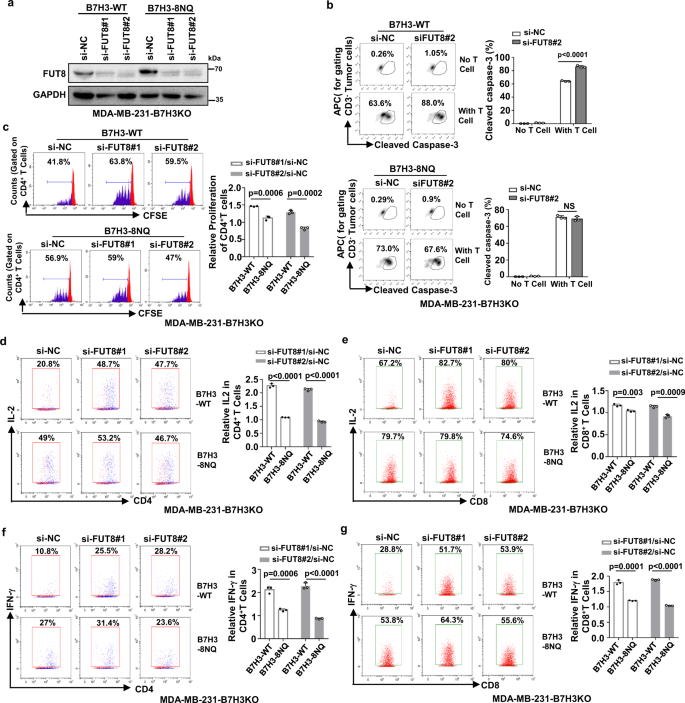
Next, the role of FUT8 knockdown in TNBC cell immune response was investigated. It has been found that FUT8 knockdown in TNBC cells enhances proliferation and viability of T cells, and fucosyltransferase FUT8 mediates aberrant N-glycosylation of B7H3 and suppresses the immune response in triple-negative breast cancer.
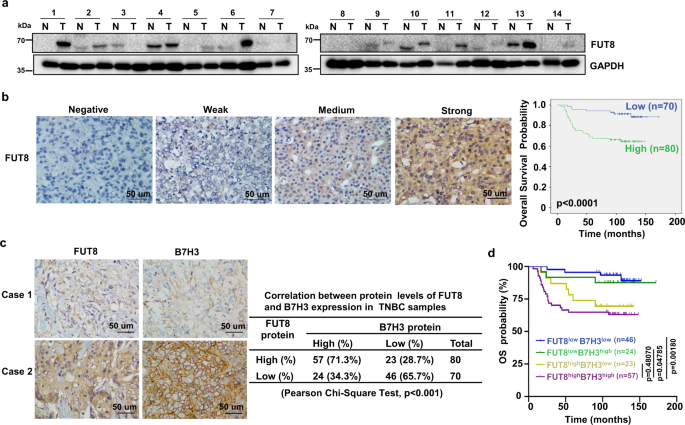
Later, the clinical significance of FUT8-mediated B7H3 glycosylation in TNBC patients has been evaluated, which was demonstrated to be physiologically significant and clinically relevant in TNBC patients, and FUT8 expression may be a main determinant for poor prognosis.
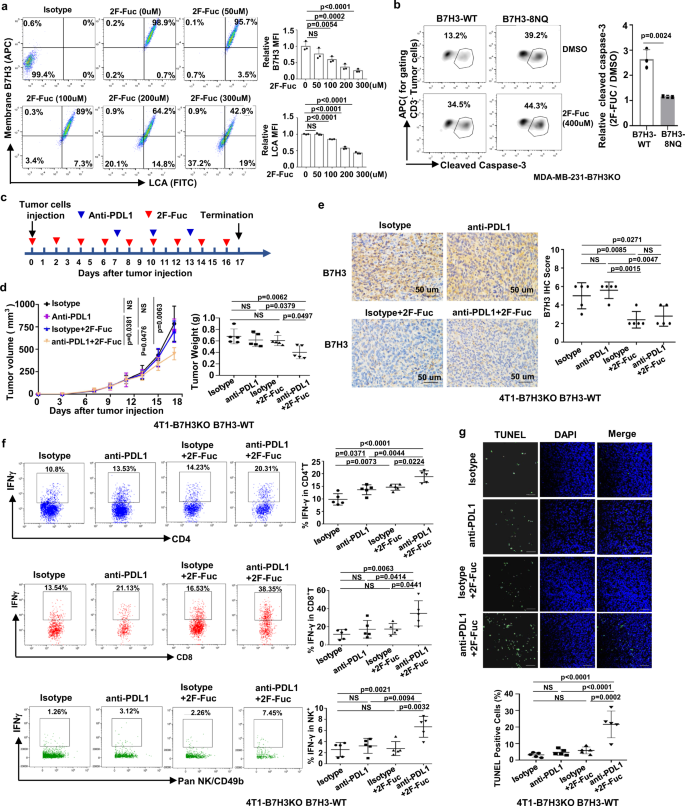
Ultimately, researchers corroborated that 2F-Fuc blocking B7H3 core fucosylation to treat B7H3-positive TNBC has potential to potentiate the efficacy of PDL1 immunotherapy.
The above is the overall process of this research.In addition, we also sort out some glycosylation-related literature available for consultation if interested.
1) Protein glycosylation: a promising way to modify the functional properties and extend the application in food system 2) N-linked glycosylation and homeostasis of the endoplasmic reticulum 3) Glycosylation and Protecting Group Strategies Towards the Synthesis of Saponins and Bacterial Oligosaccharides: A Personal Account 4) Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey 5) Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity 6) O-Glycosylation methods in the total synthesis of complex natural glycosides 7) Glycosylation defects, offset by PEPCK-M, drive entosis in breast carcinoma cells 8) Sweet Talk: Protein Glycosylation in Bacterial Interaction With the Host
|