Please click the button below to go to our email login page
|
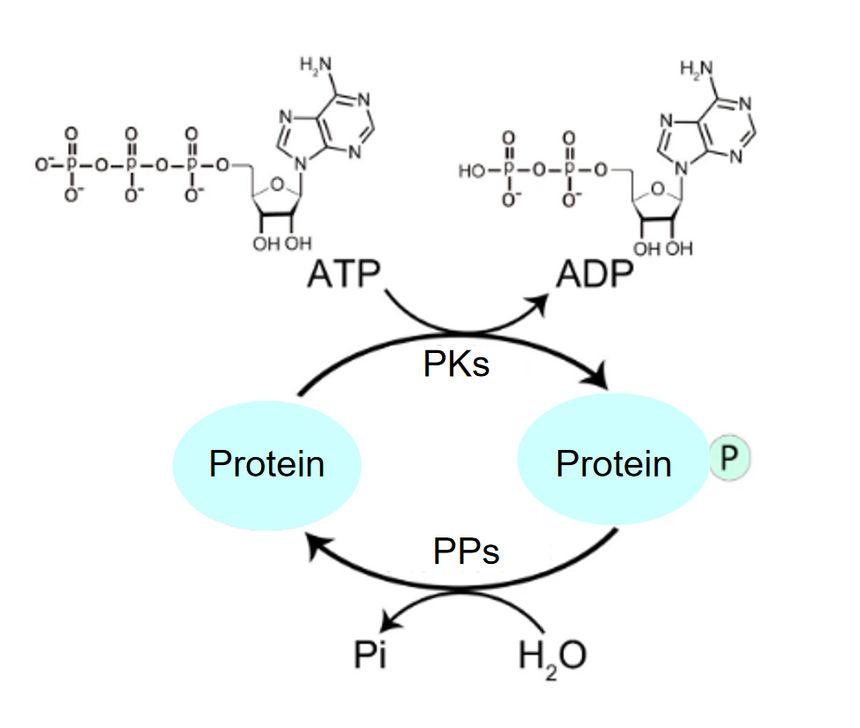
Here is the answer for how to conduct research on protein phosphorylationProtein phosphorylation is a process where phosphate group is transferred from one compound to another by enzymatic reaction, playing a pivotal role in cell signal transduction. Phosphorylation is instrumental in mediating protein functions. As depicted in the following figures, phosphorylation is a process of transferring γ phosphate groups of ATP or GTP to substrate protein on amino acid residues under catalysis of protein kinases, while the reverse process is the removal of the corresponding phosphate group by protein phosphatases.
The following is one literature with a high IF. Let’s learn how to combine protein phosphorylation into your own research.
Lung cancer is one of the deadliest malignancies over the years, whose high mortality rate mainly results from easy metastasis. Hence, in-depth research on the pathogenesis of lung cancer metastasis is necessary for control and treatment of lung cancer.
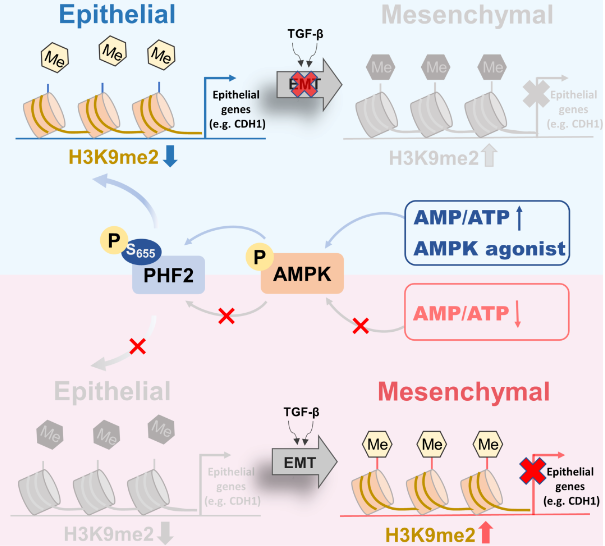
Epithelial to mesenchymal transition (EMT) is critical in cancer metastasis, accompanied by significant epigenetic changes. AMP-activated protein kinase (AMPK), a cellular energy sensor, plays regulatory roles in multiple biological processes. Despite some research illuminating the mechanism of AMPK regulating cancer metastasis, the internal epigenetic mechanisms remain poorly defined.
In this research, the effect of AMPK on lung cancer progress has been firstly determined. Based on this, this study further investigates the interplay between PHF2 and AMPK and their impacts upon lung cancer cells, whether AMPK-mediated PHF2 phosphorylation participates in regulation of lung cancer progress, and how PHF2 impacts the anti-metastasis effect of metformin, so as to unveil the prognosis significance of PHF2 on lung cancer patients.
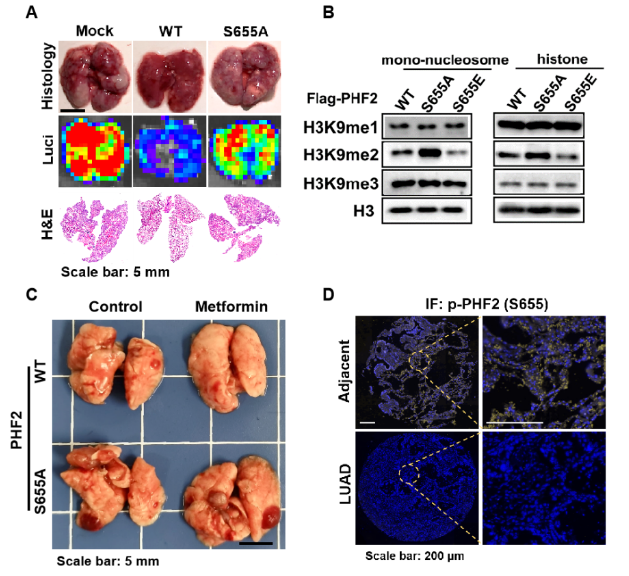
The research found that metformin-activated AMPK can suppress lung cancer metastasis of mice and down-regulate H3K9me2 level. PHD finger protein 2 (PHF2), a histone demethylase acting on H3K9me2 sites, is a new downstream phosphorylation substrate of AMPK. AMPK phosphorylates PHF2-S655 sites to mediate its histone demethylase activity and decrease H3K9me2 enrichment in the promoter region of the downstream target gene, CDH1, thereby inhibiting EMT occurrence. Besides, AMPK-mediated PHF2-S655 phosphorylation level is evidently reduced in lung cancer clinically.
This research unraveled new substrates and mechanisms of AMPK dampening lung cancer metastasis in the field of epigenetics, and identified PHF2-S655 phosphorylation as the potential biomarker in lung cancer metastasis, which contributes to expanding AMPK protein regulatory network, promoting the clinical application of metformin and providing new targets for lung cancer metastasis and deterioration.
The new mechanism of AMPK epigenetically modulating H3K9me2 to inhibit lung cancer metastasis
When exploring the regulatory effect of AMPK-mediated PHF2 phosphorylation on lung cancer progression, the author hypothesized PHF2 as the phosphorylation substrate of AMPK kinase, and identified this hypothesis through a series of assays. Then, exploration regarding the impact of phosphorylation on PHD has been performed, the results of which showed that phosphorylation can enhance the H3K9me2 demethylation activity of PHF2 and promote PHD domain to bind with H3K4me3. Ultimately, the biological function of PHF2-S655 phosphorylation on lung metastasis has been confirmed, that is, PHF2-S655 can further enhance the anti-metastasis effect of PHF2.
Through teasing out the above research process, we can understand the four basic steps of protein phosphorylation research: 1. Determine whether target protein exists phosphorylation 2. Identify phosphorylation sites 3. Analyze the impact of phosphorylation on target protein 4. Explore the biological function of target protein phosphorylation
In addition, we also have organized some phosphorylation-related reports, which are available for free if interested.
1)Dissecting the role of protein phosphorylation: a chemical biology toolbox. 2)Negative regulation of NF-κB p65 activity by serine 536 phosphorylation. 3)Phosphorylation of Ku70 subunit by cell cycle kinases modulates the replication related function of Ku heterodimer. 4)Chemical Approaches to Investigate Labile Peptide and Protein Phosphorylation. 5)A review on kinases phosphorylating the carboxyl-terminal domain of RNA polymerase II-Biological functions and inhibitors. 6)Metal/ADP Complexes Promote Phosphorylation of Ribonucleotides. 7)Histidine phosphorylation in human cells; a needle or phantom in the haystack? 8)Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. |