Please click the button below to go to our email login page
|
How to crack the research project design regarding “fatty acid metabolism”? Here is the answer.How to crack the research project design regarding “fatty acid metabolism”? Today, let’s read a paper with a high IF of 11.21, looking forward to sparking everyone’s scientific research inspiration.
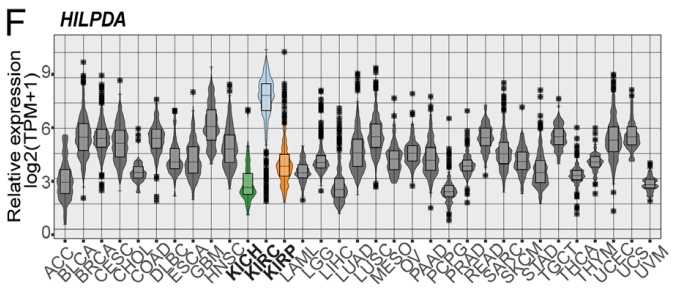
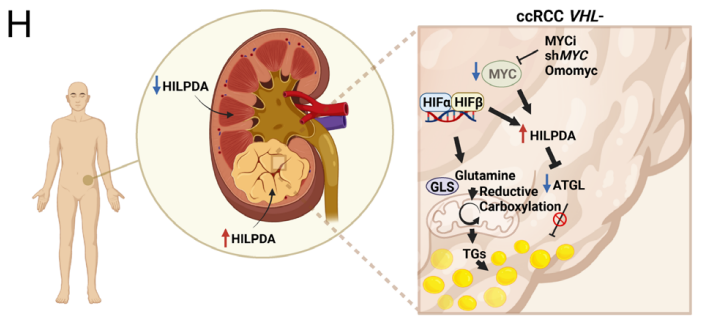
Research background: The metabolic reprogramming is paramount in tumorigenesis of clear cell renal cell carcinoma (ccRCC), which is manifested as lipid droplet (LD) in organelle. Suppression of MYC in ccRCC with VHL deficiency can glutamine-dependently increase the content of triglycerides and promote formation of LD. Besides, HILPDA has been demonstrated as the key molecule for MYC-driven LD accumulation. The comparison between ccRCC and normal samples revealed that HILPDA may be biomarker of ccRCC.
Research idea: (1) Verifying whether MYC suppression can enhance lipid accumulation based on the responses of VHL+/- cells to MYC suppression, the impact of HIF-1alpha stability on MYC and the roles of glutamine and glutaminase in MYC. (2) Analyzing the differential genes in lipid metabolism of VHL+/- cells with MYC suppression, and confirming the role of HILPDA in MYC suppression via in vitro and in vivo samples. (3) Dissecting the value of the differential gene HILPDA based on single-cell sequencing and database
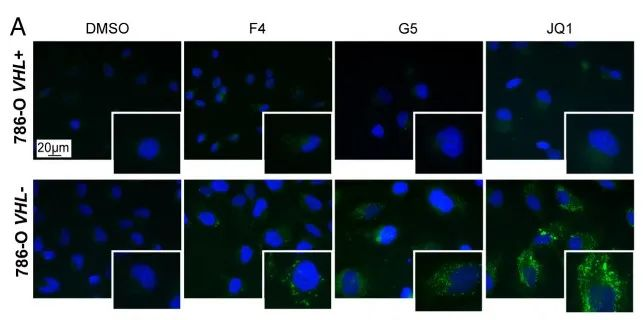
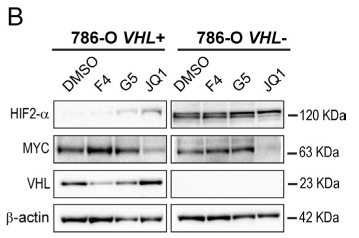
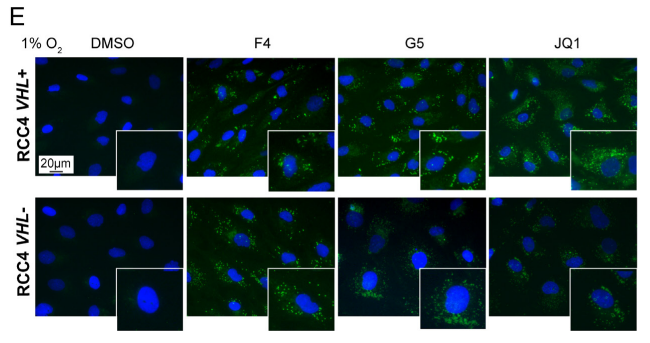
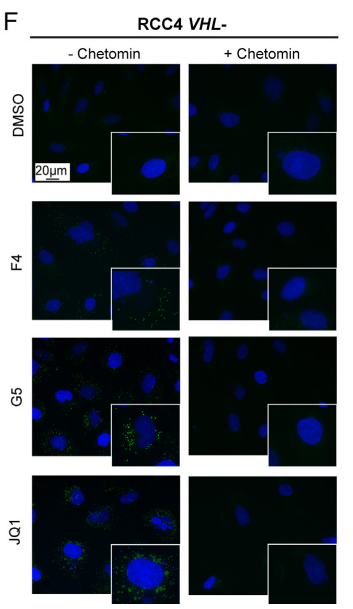
Research results: 1.The results proved that Lipid Droplet-Benzothiadiazole 1 (LD-BTD1) staining did not impact cell viability and expressions of MYC and HIF-alpha. Hence, the effect of MYC silencing on LD accumulation and its mechanism were explored. The data revealed that HIFs induced lipid accumulation in both VHL+ and VHL- ccRCC cells in the presence of MYCis, which can be prevented by HIF inhibition, hinting that MYC inhibition can boost LD accumulation through mediating HIF-1alpha in ccRCC.
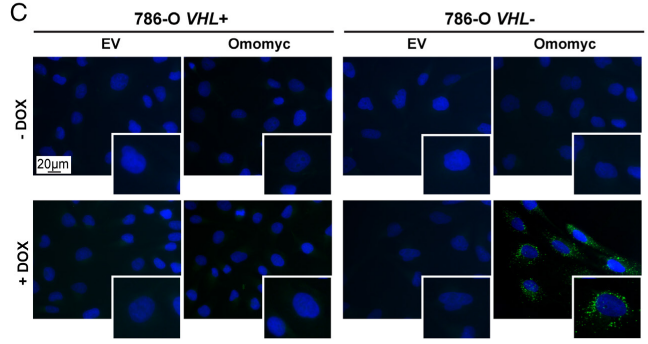
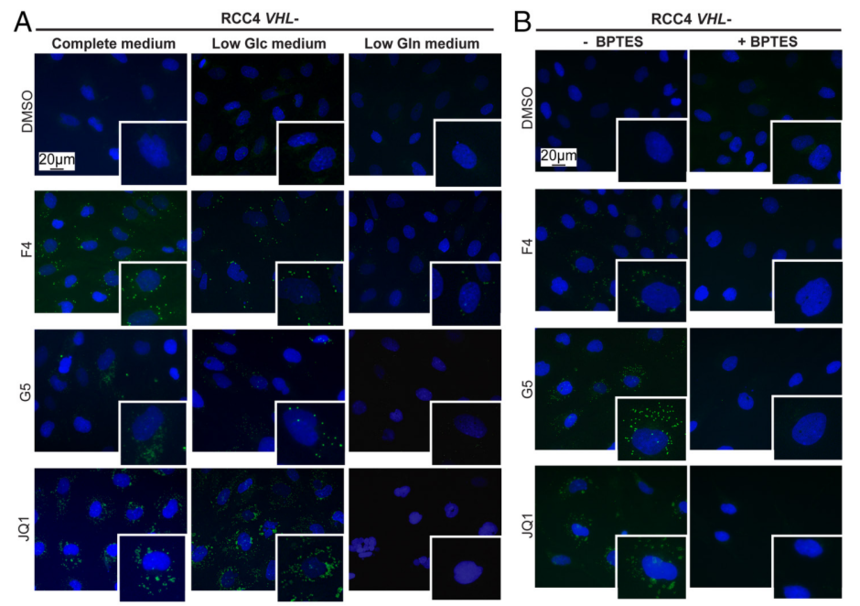
2.Then, the fuel used for LD formation was investigated. It has been demonstrated that low glutamine medium and inhibition of glutaminase can prevent MYC inhibition-induced LD accumulation. Meanwhile, Omomyc-induced lipid accumulation was repressed by inhibition of glutaminase. Collectively, glutamine was essential for fatty acid synthesis and survival after MYC knockdown.
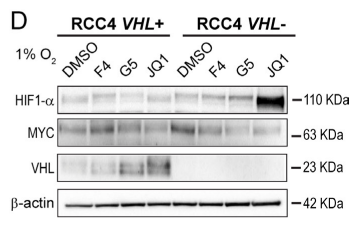
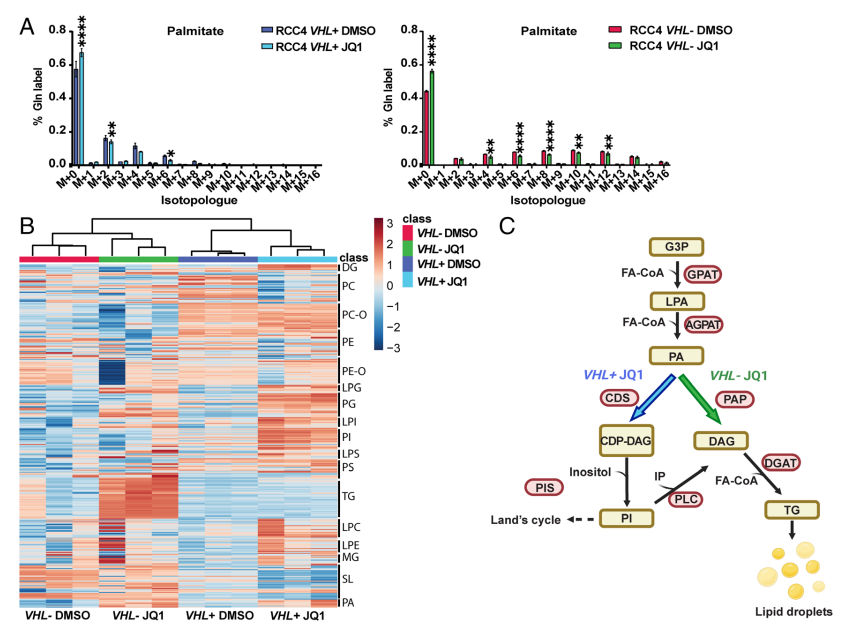
3. To fathom out whether glutamine-derived carbons were directed to fatty acid synthesis, stable isotope tracing experiments in RCC4 VHL+ and VHL− cells were performed. The results showed that MYC inhibition was detected when different lipid species were accumulated in VHL+ cells and VHL− cells.
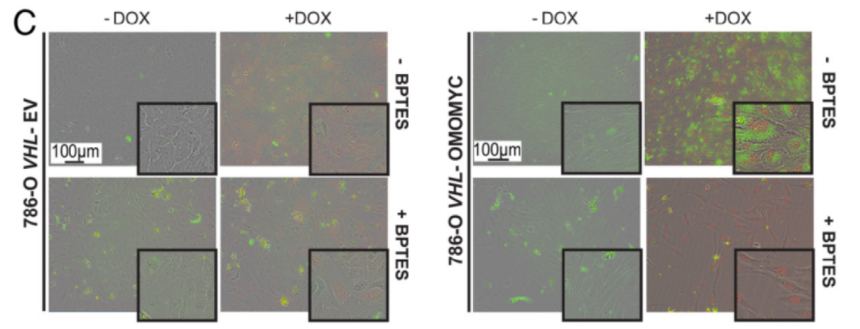
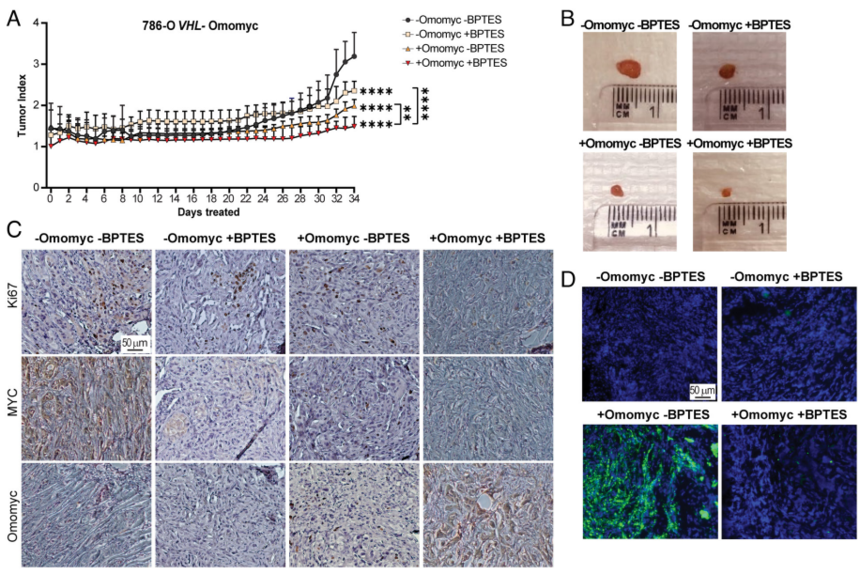
4.To analyze the correlation of combined MYC and GLS inhibition on LD accumulation as well as on tumor growth in vivo, tumor-bearing experiments were conducted. The results found that MYC inhibition without HIF suppression resulted in more lipid accumulation in tumors, while MYC and HIF inhibition caused smaller tumors, owing to the proliferation-promoting effects of MYC and HIF. Accordingly, combined inhibition of MYC signaling and glutamine metabolism led to reduced tumor growth.
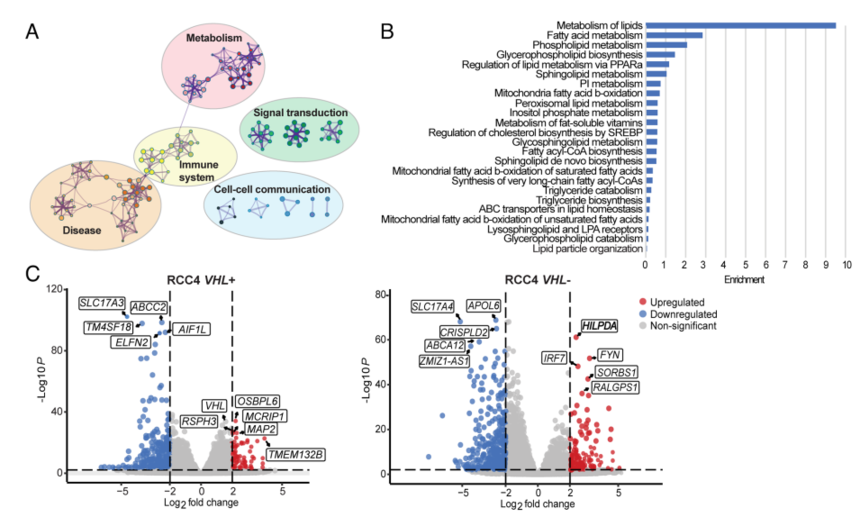
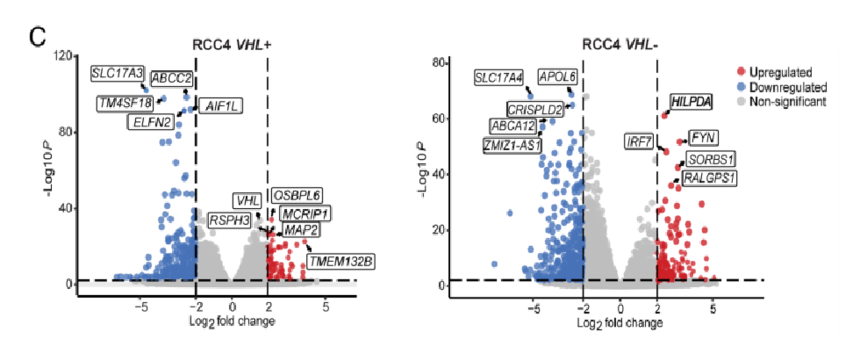
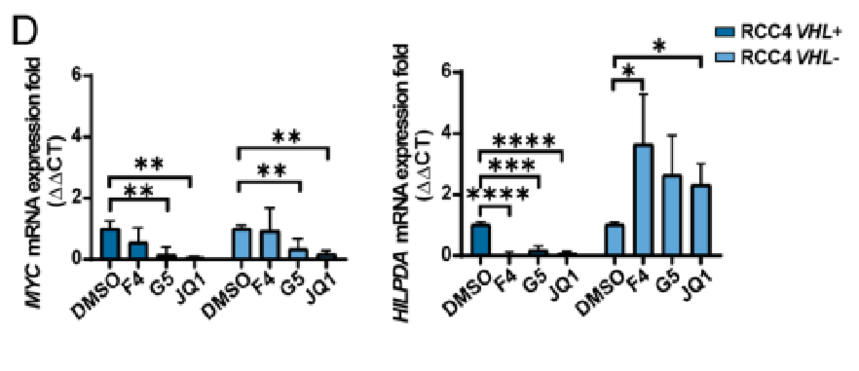
5.To ascertain the molecular mechanism of LD accumulation after MYC inhibition in HIF-expressing cells, bulk RNA sequencing (RNA-seq) analyses were carried out, which unveiled that LD-associated protein is up-regulated in VHL− cells.
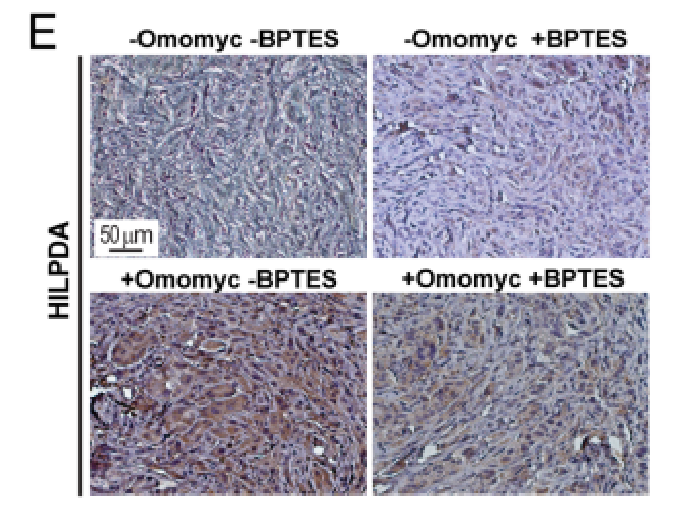
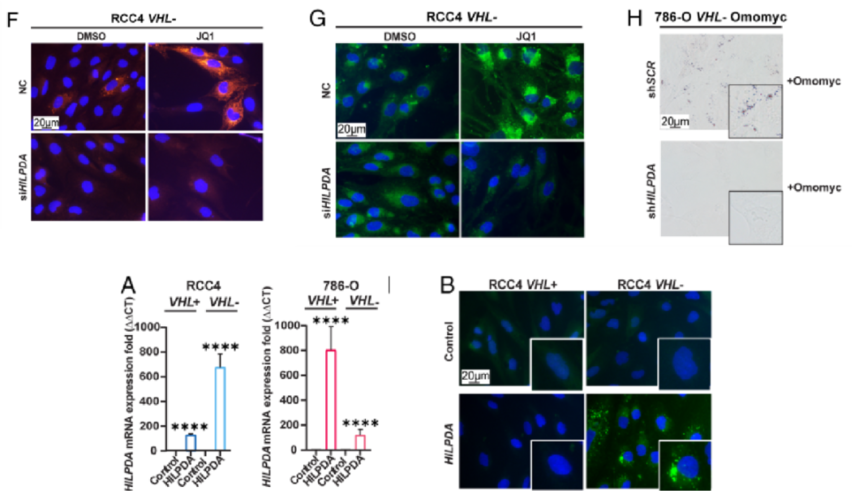
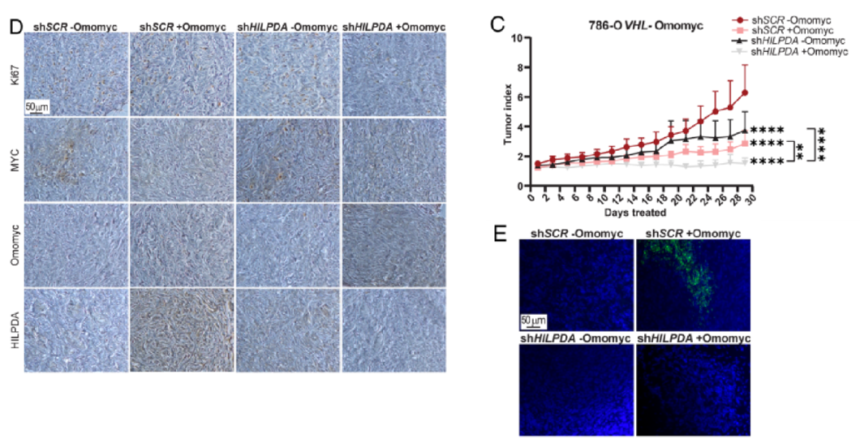
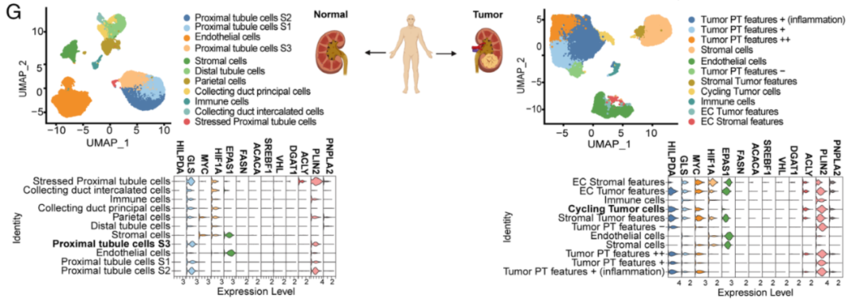
6. The study probed into whether HILPDA expression was required for inducing LD accumulation upon MYC inhibition and the impact of HILPDA down-regulation on tumor growth in vivo and LD accumulation. Besides, the study analyzed single-cell RNA-seq (scRNA-seq) from patient-derived normal kidney as well as tumor tissue and the possibility that the research results could translate to clinical samples. Ultimately, the selective expressions indicated that HILPDA provides a novel easily treatable route for ccRCC.
|