Please click the button below to go to our email login page
|
17 IF! Shixuan Wang’s group in Huazhong University of Science and Technology unlocks the space-time password of ovarian senescence: the key transcriptional regulation of FOXP1Do you know the secret of ovarian senescence? The phenomenon of women experiencing hypoovarianism before the age of 40 is termed premature ovarian failure (POF). So what are the design ideas for POF? Today, we are going to share a paper published on Nature Aging, with IF of 17, entitled Spatiotemporal transcriptomic changes of human ovarian aging and the regulatory role of FOXP1. This paper combined bioinformatics analysis and experimental verification, hoping to inspire you from different aspects.
1. Research background 1.1 Human ovary consists of different stages of follicles as basic functional units and plentiful stromal cellular elements. 1.2 In recent years, research using scRNA-seq indicated that human ovary contains abundant cell types, such as granulosa cells (GCs), oocytes, stromal cells and immune cells. 1.3 Human ovary displays extensive variation in the cortex and medulla, oogenesis starts in the cortex, and the medullary region of the ovary undergoes dramatic restructuring. 1.4 Spatial transcriptomics (ST) is a new technology that leverages the messenger RNA of cell sections using numerous barcoded oligo-dT primers and then maps transcripts to the tissue slice, enabling the spatial visualization of gene expression.
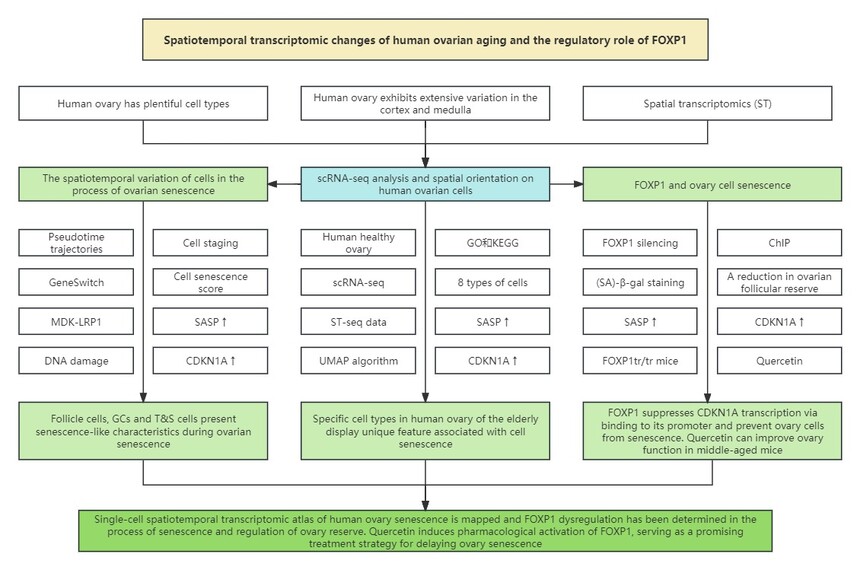
2. Technical route
3. Research results 3.1 scRNA-seq analysis and spatial orientation of human ovary cells 3.2 Cell specific changes during ovary senescent transcription 3.3 Spatiotemporal changes of oocytes during ovary senescence 3.4 Spatiotemporal transcription changes of GCs during senescence 3.5 Spatiotemporal changes of T&S cells during senescence 3.6 FOXP1 is the core transcriptional regulator of ovarian cell senescence in vitro 3.7 FOXP1 knockdown in GCs accelerates ovary senescence 3.8 Quercetin protects ovary reserve in middle-aged mice
4. Research conclusion This research observes the spatiotemporal molecular characteristics of 8 types of ovarian cells during the senescent process. Analysis of age-related changes in gene expression suggests that DNA damage may be a key biological pathway in oocyte senescence. Additionally, the study identified 3 GC subtypes and 5 zona pellucida and stromal cell subtypes, as well as their spatiotemporal transcriptomic changes during senescence. FOXP1 is a regulatory factor of ovarian senescence, which decreases with age and inhibits the transcription of CDKN1A. FOXP1 silencing leads to premature ovarian failure in mice. These findings comprehensively confirm the spatiotemporal variability of human ovarian senescence, contributing to determining the priority of potential diagnostic biomarkers and therapeutic strategies. |