Please click the button below to go to our email login page
|
New ideas in “Mitochondrial homeostasis”!Successfully published in SCI of 10.7! Unveiling the mechanism of Slc25a3-dependent copper transport on mitochondria.Mitochondria are the energy centers of eukaryotic cells and are involved in numerous cellular biological processes. They require multiple layers of quality control systems to ensure their normal function, thereby maintaining the homeostasis of cells and organisms. So what are the design ideas for mitochondrial homeostasis? Today, let’s explore together.
Next, we’re going to share a paper published on Developmental Cell, with an IF of 10.7, hoping to bring different inspirations.
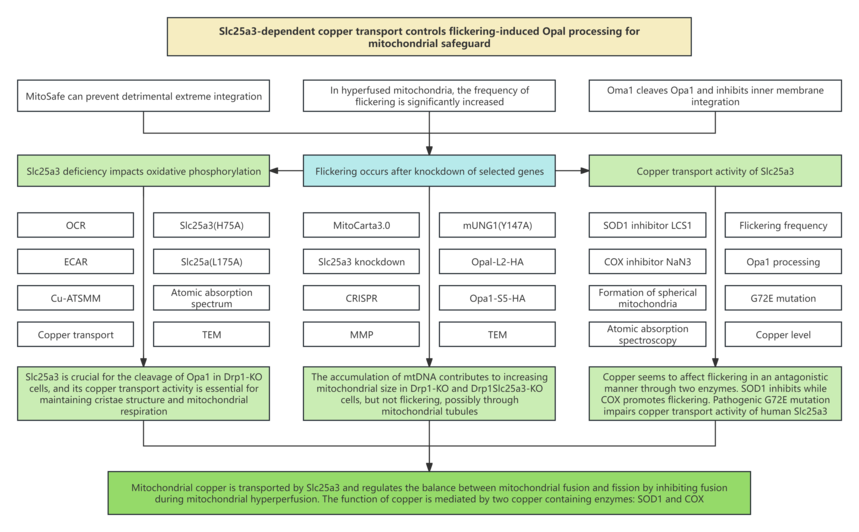
1. Research background 1.1 Many human diseases and abnormal mitochondrial structure are related to energy deficiency, aberrant distribution and autophagy transport damage. Just like the story of blonde girl and three bears, mitochondria need to be “just right”, neither too small nor too large. 1.2 MitoSafe can prevent detrimental extreme integration that can damage the respiratory functions of the outer and inner membranes of mitochondria. On the outer membrane of cells, Parkin, phosphorylated Parkin and ubiquitinated PINK1 promote proteasome degradation. 1.3 On the inner membrane of cells, Oma1 cleaves Opa1 and inhibits inner membrane integration. In MitoSafe, Oma1 is activated through repeated, brief membrane potential drops (known as flickering) without losing the entire membrane potential. 1.4 In hyperfused mitochondria, the frequency of flickering is significantly increased. Therefore, MitoSafe can be considered a key biological process for flickering function. However, it is currently unclear how prolonged mitochondria cause flickering.
2. Technical routes
3. Research results 3.1 RNA interference screening based on live-cell imaging reveals that Slc25a3 is crucial for the flickering of Drp1-KO cells. 3.2 Slc25a3 is important for enhancing the cleavage of Opa1 in Drp1-KO cells. 3.3 The simultaneous deficiency of Drp1 and Slc25a3 leads to the formation of giant granular mitochondria and aggregated mitochondrial DNA (mtDNA). 3.4 The deficiency of Drp1 and Slc25a3 affects oxidative phosphorylation and glycolysis. 3.5 Copper transport mediated by Slc25a3 is pivotal for the flickering, Opa1 processing, and mitochondrial morphology in Drp1-KO cells. 3.6 The copper-binding enzymes SOD1 and COX control flickering in opposite ways. 3.7 The pathogenic mutation G72E inhibits the copper transport activity of human Slc25a3.
4. Research conclusion In this study, we first identified that the deficiency of Slc25a3 significantly reduces the flickering frequency of Drp1-KO cells through genetic screening, and further confirmed that copper transport mediated by Slc25a3 plays a crucial role in the flickering and Opa1 processing in Drp1-KO cells. Additionally, in the deficiency of Drp1, Slc25a3-mediated copper transport affects mitochondrial morphology, energy metabolism, and mtDNA distribution. Ultimately, we revealed that mitochondrial copper is transported by Slc25a3 and regulates the balance between mitochondrial fusion and fission by inhibiting fusion during mitochondrial hyperfusion. This function of copper is mediated by two copper-containing enzymes: the copper-dependent enzyme SOD1 inhibits flickering, while COX promotes flickering to influence the flickering process. |