Please click the button below to go to our email login page
|
IF of 14.3! New advanced findings of “NF-κB signaling pathway”! Unveiling the new mechanism of NSD2 inhibiting tumorNF-κB is a key nuclear transcription factor, playing critical role in cell response to multiple stimuli. Then, what are the research ideas for the NF-κB signaling pathway?
Next, we will share a paper published on Advanced Science, with an IF of 14.3, hoping to bring you different inspirations.
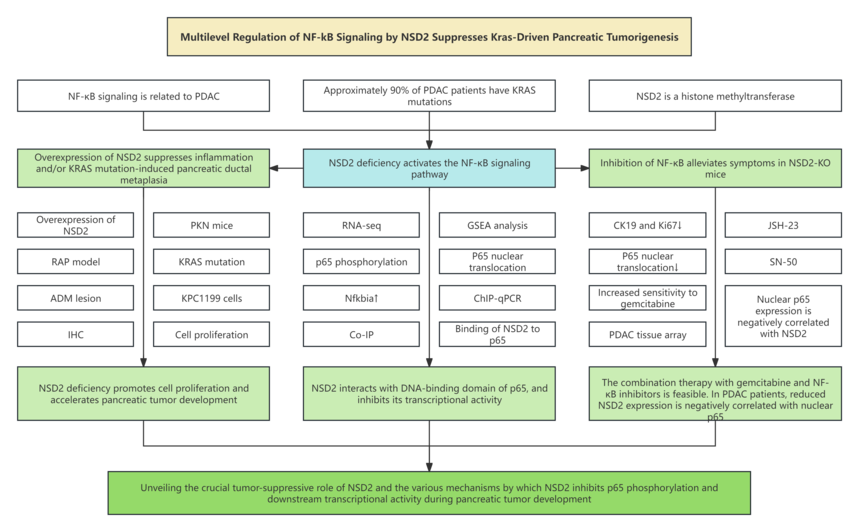
1. Research background 1.1 The occurrence and development of pancreatic tumors involve multiple genetic variations, including KRAS, TP53, SMAD4 and CDKN2A. Among them, approximately 90% of patients with pancreatic ductal adenocarcinoma (PDAC) have KRAS mutations. 1.2 The NF-κB signaling pathway is constitutively activated in nearly 70% of pancreatic cancer specimens and is instrumental in the development, progression and drug resistance of PDAC. 1.3 NSD2 is a histone methyltransferase that catalyzes the dimethylation of histone H3 at the lysine 36 (H3K36me2) site, and H3K36me2 is an allowed marker associated with transcription of active genes. 1.4 Many studies revealed that NSD2 boosts cell proliferation, migration, invasion ans epithelial-mesenchymal transition (EMT), proving that it plays a vital role in promoting cancer.
2. Technical routes
3. Research results 3.1 NSD2 overexpression suppresses inflammation and/or KRAS mutation-induced pancreatic metaplasia. 3.2 NSD2 deficiency boosts KRAS-induced ductal metaplasia. 3.3 NSD2 deficiency activates NF-κB signaling pathway. 3.4 NSD2 promotes Nfkbia expression and dampens p65 nuclear translocation. 3.5 NSD2 interacts with DNA-binding domain of p65, and inhibits its transcriptional activity. 3.6 Inhibiting NF-κB signaling transduction can alleviate the symptoms of NSD2-deficient mice, and improve the sensitivity of NSD2-deficient PDAC to gemcitabine. 3.7 PDAC patients displayed downregulated NSD2, which is negatively correlated to nuclear p65 expression.
4. Conclusion In this study, the researcher firstly analyzed clinical database, and found that compared to normal pancreatic tissues, tumor tissues of PDAC patients has downregulated NSD2 mRNA, implying that the function of NSD2 in pancreatic cancer may not be consistent with that in other cancers previously reported. Next, the researcher utilized a KrasG12D-induced pancreatic tumor mouse model and found that specific overexpression of NSD2 in the pancreas can inhibit inflammation and KrasG12D-induced acinar ductal metaplasia in mice. On the contrary, the pancreatic specific deficiency of NSD2 promotes KrasG12- induced tumorigenesis. Subsequently, it is also confirmed that NSD2 can interact with the DNA-binding domain of p65 protein, thereby directly inhibiting the downstream transcriptional activity of NF-κB. In addition, using SN50 and JSH-23 to inhibit NF-κB signaling transduction can alleviate the tumor lesion phenotype of Nsd2-deficient PDAC mice and make Nsd2-deficient PDAC more sensitive to gemcitabine treatment. Ultimately, the researcher applies clinical PDAC database and samples to further demonstrate that NSD2 level is downregulated in PDAC patients and is negatively related to nuclear p65 level. This study unravels a new tumor-suppressing mechanism of NSD2, and also provides a new theoretical approach for PDAC patients with low expression/deficiency of NSD2 to receive combined treatment with gemcitabine and NF-κB inhibitors.
|