Please click the button below to go to our email login page
|
Practical Tips! Common Issues and Solutions in Cell Thawing
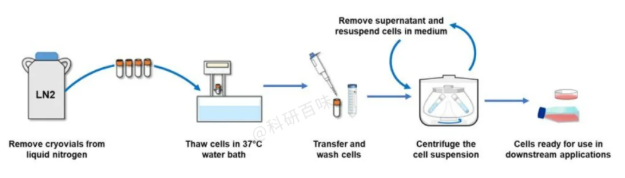
Cell thawing is a process completely opposite to cell cryopreservation, which refers to the restoration of cell growth, a core link in cell culture technology. 1. Basic steps of cell thawing 1.1 Remove cryovials from the liquid nitrogen container, directly immerse in 37℃ warm water and shake it from time to time for quick melting. 1.2 Remove cryovials from 37℃ water bath, uncap, aspirate the cell suspension with a pipette, transfer it into centrifuge tube, add more than ten times the amount of culture medium and mix well. 1.3 Centrifuge at 1000 rpm for 5 min. 1.4 Remove supernatant, add culture media containing 10% fetal bovine serum for resuspending cells, count, regulate cell density, inoculate into culture flask, and incubate in 37°C incubator 1.5 Refresh culture media the next day and continue the culture.
2. Common problems in cell thawing 2.1 Low cell viability or cell death [Possible reasons] l Cryoprotectant of poor quality or inappropriate proportion (e.g. no pre-cooling or contamination of DMS0) l Poor cell status before cryopreservation (e.g. higher passage, not in logarithmic growth phase) l Slower thawing speed or dramatic changes of temperature that cause ice crystal damage l Inappropriate cryopreservation procedure (e.g. directly put into liquid nitrogen without program cooling box)
[Solutions] l Select high-quality cryoprotectant (e.g. pre-cool serum-containing or serum-free cryoprotectant), and avoid higher local concentration of DMSO l Cryopreserve healthy cells in logarithmic growth phase (nearly 80% confluency of adherent cell, and >90% viability of suspension cells) l Rapid thawing: immerse cryovials into 37℃ water bath and gently shake it for melting completely within 1 to 2 minutes l Use program cooling box (reduce to -70℃ at the speed of 1°C/min and then transfer and store in liquid nitrogen)
2.2 Difficulty in cell adhering to wall [Possible reasons] l Excessive digestion before cryopreservation (pancreatic enzyme functioning too long or with too high concentration) l Too high centrifugation speed or too strong blowing force after thawing, resulting in cell breakage l Inappropriate culture conditions (e.g. incorrect CO2 concentration, unheated culture medium) l Microbial contamination ((e.g. water infiltration into cryovials during water bath)
[Solutions] l Control digestion time before cryopreservation, and immediately terminate digestion when over 80% cells exfoliate. l Low-speed centrifugation (250~1000 rpm for 3~5 minutes) and gently blow the cell suspension. l Ensure that the culture medium is matched with the cell type, pre-warm to 37℃, and check the CO2 concentration. l Conduct strict sterile operations: wrap the cryovial with PE gloves during water bath, and wipe the mouth of the tube with alcohol after thawing.
2.3 Slow or stagnant cell growth after adhesion [Possible reasons] l Low cell density during cryopreservation or cells entering senescence l Incomplete removal of DMSO (highly toxic to some sensitive cells) l Genetic engineering impacts (e.g. essential knockout or overexpression gene products inhibiting proliferation)
[Solutions] l Adjust cell density to 5×10⁵~1×10⁶ cells/mL during cryopreservation, and avoid using high-passage cells. l Remove DMSO from the cryopreserved medium by centrifugation, or directly dilute 10 times before adhering and changing the medium. l For genetically engineered cells (e.g. knockout/overexpression strains), assess gene necessity or change the host cells.
2.4 Cell contamination [Possible reasons] l Incomplete sealing of cryovials, leading to liquid infiltration during water bath l Contaminated operating environment or reagents (e.g. mycoplasma, bacteria)
[Solutions] l Check the cryovial cap for proper sealing before cryopreservation, and avoid the water level towering over the tube mouth during water bath l Regularly test cells and reagents for microbial contamination, and fumigate the laboratory with hydrogen peroxide periodically.
2.5 Operational errors [Possible situations] l Failure to process cells promptly after thawing (prolonged retention of cryopreservation medium) l Temperature fluctuation during transportation (e.g. failure to use dry ice or cold packs for insulation)
[Solutions] l Centrifugation or dilution immediately after thawing to minimize the exposure time of DMSO l Use dry ice or insulated containers to maintain low temperatures when transporting cryovials over long distances |