Please click the button below to go to our email login page
|
Determination of the Optimal MOI for Cell Infection (Commonly Asked Questions and Answers Appended)Multiplicity of infection (MOI) refers to the ratio of viruses and cells during infection, the implicit unit of which is pfu number/cell. However, the number of some viruses, such as AAV, is expressed by TU, IU, viral particles (v.p) or vector genome (v.g.), instead of pfu.
Lentivirus vector, a single-stranded RNA virus vector based on HIV-1, can infect dividing or non-dividing cells, and effectively integrate exogenous genes into the host’s chromosome, which has relatively low immunogenicity and has been widely applied in various in vitro cell experiments.
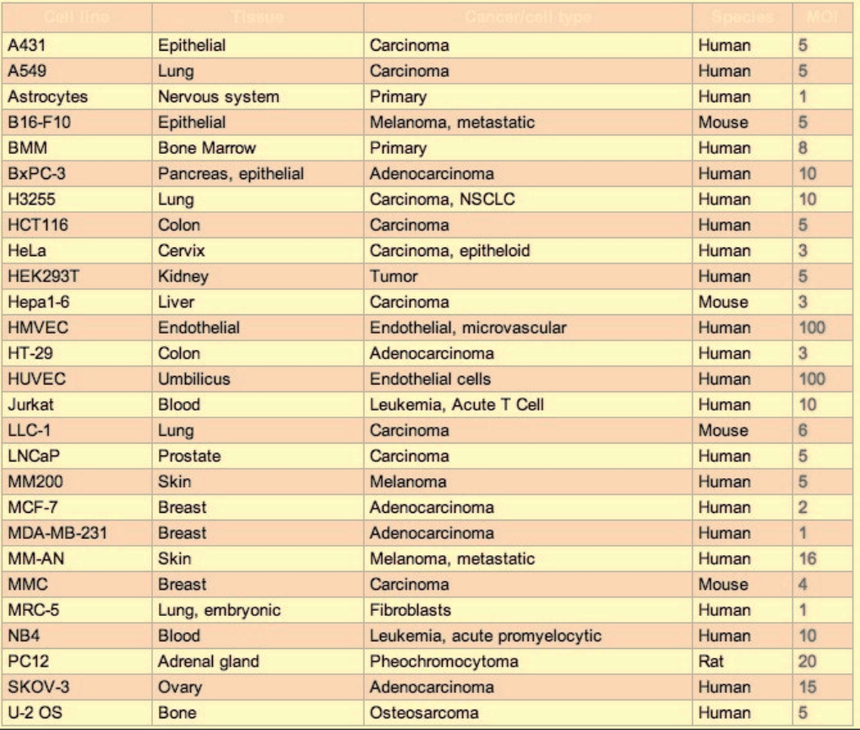
Reference value of common cell MOI (Lentivirus)
Steps to explore MOI value of lentivirus 1. Pre-experimental information preparation 1) Culture condition of target cell line and proliferation rate 2) Exclude cell mycoplasma contamination 3) Search literature and obtain reference value of MOI of target cells (if no relevant literature exists, a pre-experiment can be conducted first by setting a MOI with a larger gradient)
2. MOI gradient setting 1) Two gradients are set before and after the searched reference MOI value, with each gradient differing by at least twice 2) Calculate the required virus load according to the formula: MOI value = virus titer (TU/mL) × virus volume (mL)/the number of cells
3. Planting cells 1×104 cells/well in a 96-well plate with 100 μL/well culture media
4. Lentivirus infection As per the calculated virus volume, each well is added with lentivirus and the culture media are refreshed on the day following infection.
5. Determination of MOI range 1) Lentivirus with fluorescent tags: fluorescent observation. 72 h after infection, cell morphology and fluorescence are observed under fluorescent microscope, determining the well with relatively good cell morphology and more fluorescent count to obtain appropriate MOI value. 2) Lentivirus without fluorescent tags: resistance screening (puromycin/blasticidin, etc.). Refer to the appropriate screening concentration of the resistant antibiotic in the target cell line, add the drug 72 h post-infection, culture for 3-5 days, and examine under the microscope. Choose the wells showing high cell viability and good morphology to obtain the corresponding appropriate MOI value.
6. Narrow the MOI range for a second round of exploration (additional steps) If a more precise MOI value is required, set up gradient MOI within the range of MOI identified in step (5) and repeat the procedures described in steps (3)–(5).
Commonly asked questions regarding lentivirus infection
1) How can the infection efficiency of lentivirus be improved? Good growing status of cells is the basis of high infection efficiency. If necessary, the infection-assisting reagent ADV-HR can be assed during infection to enhance infection efficiency of lentivirus.
2) What are the working concentration and application protocol of the infection-assisting reagent ADV-HR? The infection-assisting reagent ADV-HR can significantly enhance infection efficiency of lentivirus in dose-dependent and time-dependent manners. But higher concentration of ADV-HR has cytotoxicity that impacts cell status and infection efficiency, so it is recommended to conduct a pre-experiment of ADV-HR concentration gradient in the target cells.
3) Why do numerous black dots appear in the cells after lentiviral infection and compromise cell growth? After ruling out the possibility bacterial and fungal contamination, the black dots in the cells may be cell debris with many causes, but after lentivirus infection, there are two common reasons for cell debris: Overuse of lentivirus or too few cells; Mycoplasma contamination. Mild mycoplasma contamination is often neglected because it does not overtly inhibit cell growth or proliferation; however, mycoplasma undergoes explosive growth after viral infection, producing extensive cell debris. Testing the cells, culture reagents, and incubation environment for mycoplasma is strongly recommended before using any viral products to avoid wasting time. |