Please click the button below to go to our email login page
|
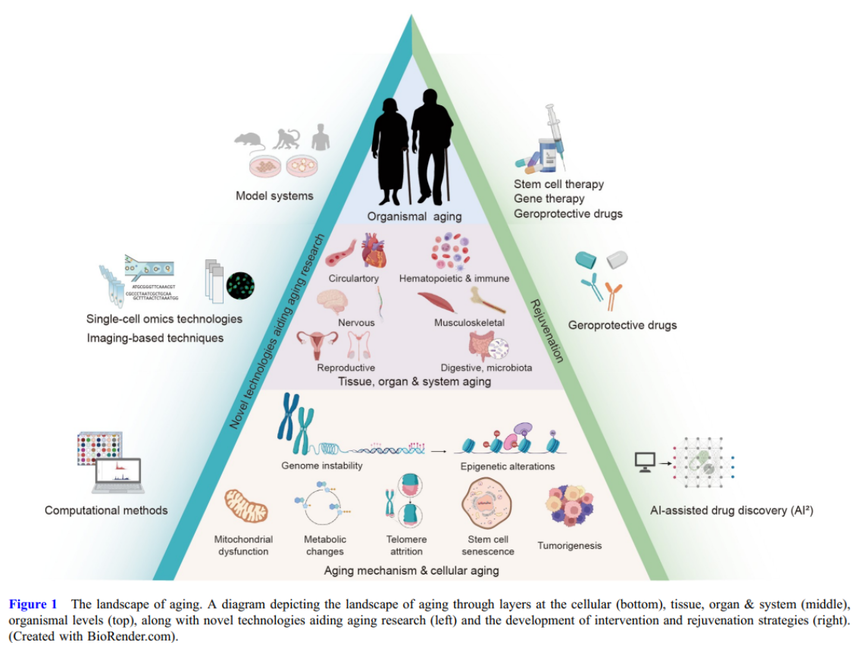
Focusing on senescence: Guanghui Liu’s team continuously makes breakthrough in 2025!Senescence is a classical, irreversible form of proliferation arrest, which may be a protective mechanism for maintaining tissue homeostasis and a kind of supplementary mechanism for programmed cell death, thereby timely removing diseased, dysfunctional or other unnecessary cells. Beyond closing cell cycle, senescent program also can induce changes in cell morphology and metabolism, the most prominent characteristic of which is the activation of the senescence-associated secretory phenotype (SASP) and the release of substantial bioactive proteins.
The professor Guanghui Liu from Chinese Academy of Sciences has long been conducting research on senescence, focusing on decoding senescent mechanism and developing intervention strategies to achieve healthy senescence. Throughout his scientific career, Professor Liu has made exceptional achievements in the senescent research field: from decoding hereditary code of senescence, to develop stem cell intervention strategies, and to promote precise gene treatment. He constructed a complete, cutting-edge research system.
The research system of Guanghui Liu’s team is a complete closed cycle, “understanding mechanism”-“establishing models”-“developing therapy”, where two core technologies, stem cells and gene editing, are utilized, with the ultimate goal of achieving effective intervention in human senescence and healthy aging. Guanghui Liu’s team has published multiple achievements in top-tier journals such as Cell, Nature, and Science, with citations exceeding 20,000, and Professor Liu has been recognized as a highly cited Chinese researcher. In 2025, Guanghui Liu’s team published three landmark papers in the journal Cell, systematically elucidating the core mechanism of senescence and providing intervention roadmap that can be clinically translated.
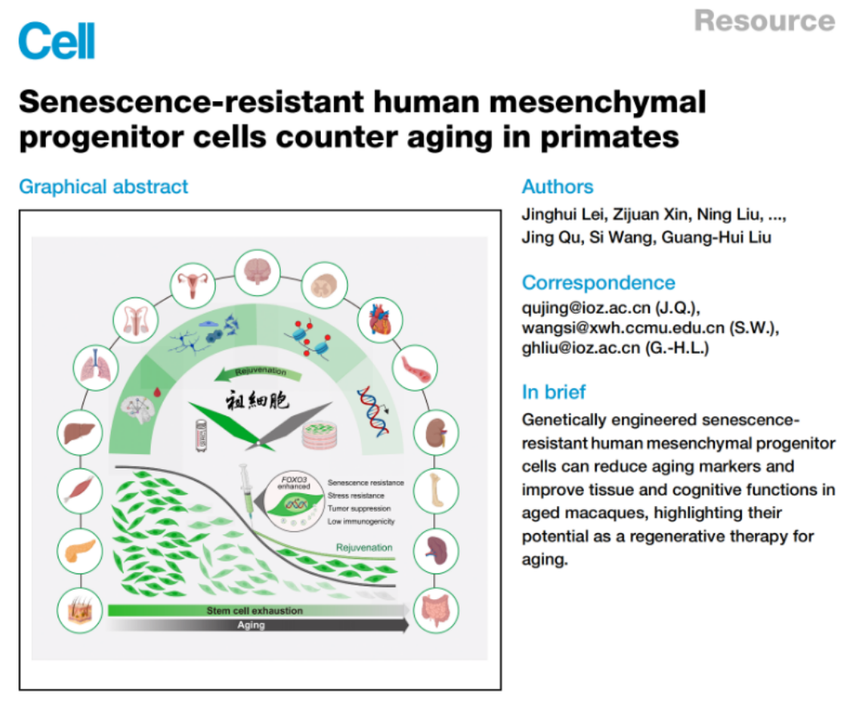
In September 2025, the paper “Senescence-resistant human mesenchymal progenitor cells counter aging in primates” was published in the top-tier journal Cell, which is a landmark research systematically discussing stem cell intervention in primate senescence. This research developed human mesenchymal progenitor cells with anti-senescence effects, which apparently decelerated senescence of primates via enhancing FOXO3 activity, and utilized CRISPR-Cas9 to conduct FOXO3 gene editing in human embryonic stem cells (S253A/S315A amphimutation) and ultimately establish anti-senescent phenotype.
In June 2025, the research “Systematic profiling reveals betaine as an exercise mimetic for geroprotection” firstly found that betaine is a kind of “exercise mimetic”, possessing anti-inflammatory and geroprotection effects. Through recruiting 13 healthy males and collecting blood and fecal samples, multi-dimensional analyses including single-cell transcriptomics, plasma proteomics, metabolomics, and microbiome analysis were performed. The mouse model was employed to verify the mechanism of betaine in exercise mimetic. The research systematically revealed that betaine, as a potential exercise mimetic, plays anti-inflammatory, anti-oxidative and anti-senescent roles via inhibiting TBK1 signaling pathway, thus providing theoretical basis and practical routes for developing “exercise pills”.
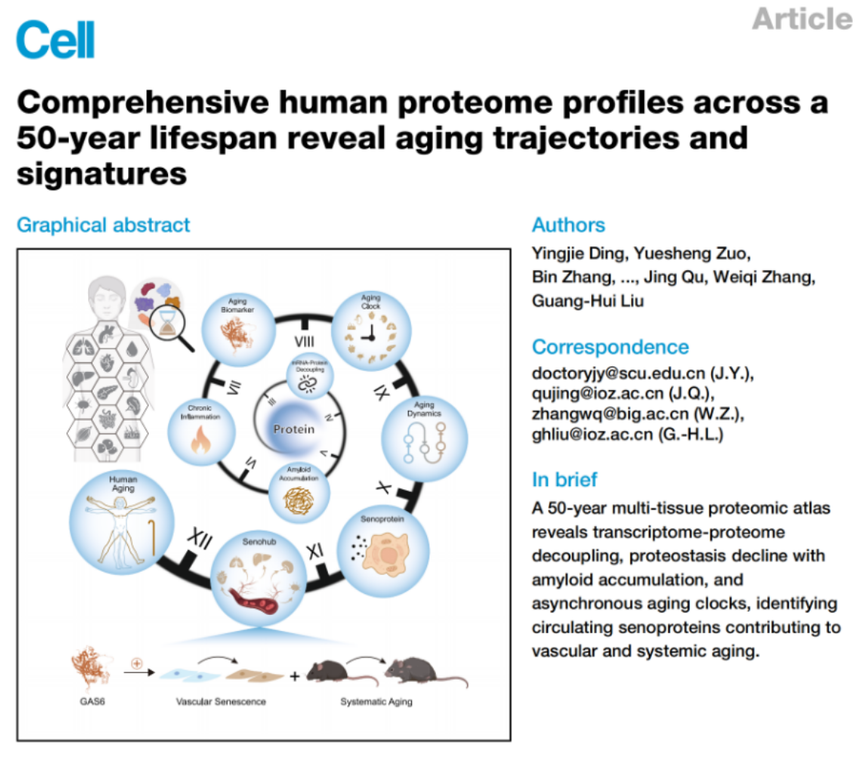
The research titled “Comprehensive Human Proteome Profiles Across a 50-Year Lifespan Reveal Aging Trajectories and Signatures” integrated ultra-sensitive mass spectrometry technology with machine learning algorithms for the first time, and systematically constructed a proteomic senescent atlas spanning 50 years of human life, covering 7 physiological systems and 13 key tissues, which presents a panoramic dynamic landscape of age-related physiological changes from a protein perspective. This “chronicle of senescent protein molecular in humans” revealed that the disruption of protein information flow is one of the core characteristics of organ senescence, the essence of which lies in the synergistic effects of mRNA-protein uncoupling and pathological amyloid deposition that lead to systemic disintegration of the protein homeostasis network. Meanwhile, the research has, for the first time, established the vascular system as the “pioneer tissue” of the senescent process, which evidently deviates from homeostasis trajectory in the early stage of life. The senescent blood vascular specifically secretes GAS6 and other pro-senescent proteins and activates cross-organ cascade signaling networks, thus exerting central regulatory function as a “senescence hub”, and driving and amplifying the senescent process in multiple organ systems throughout the body. |